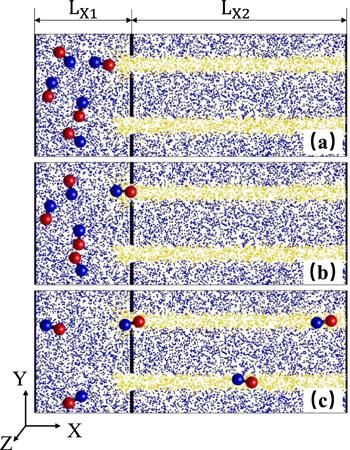
The device is designed by a container and a microchip including chemical pathways, as shown in figure
1. In the container, nanodimers initially diffuse in the liquid solution which is modeled by amounts of point-like solvent particles (S). The container and the chip are bounded by a wall composed of immobile beads with the radius
Σb = 0.5, except for several entrances. In simulation, the chemical pathways are constructed as follows: when solvent particles (S) diffuse into the stripe pathways they are converted to fuel (F) particles; when molecules diffused out of the pathways they undergo the decay reaction, F (or P)
$\mathop{\longrightarrow }\limits^{{k}_{{\rm{cat}}}}{\rm{S}}$, which converts them back to solvent particles with a rate constant
kcat [
24]: once the F (or P) diffuse out of the stripe at time
t, one can compute the time of the conversion to S at
t +
td from
$t+\mathrm{ln}(1/U)/{k}_{{\rm{cat}}}$, where
U is a random number chosen from a uniform distribution on the interval [0,1). The self-propelled dimers consist of the catalytic (C) and noncatalytic (N) spheres which are linked by a fixed distance
R by a holonomic constraint [
25,
26]. The chemical reaction,
${\rm{F}}+{\rm{C}}\to {\rm{P}}+{\rm{C}}$, occurs at the catalytic sphere of the dimer with a subsequent product (P) once a fuel particle (F) touches the surface of the C monomer.