1. Introduction
2. Computational detail
3. Results and discussion
3.1. Structural properties
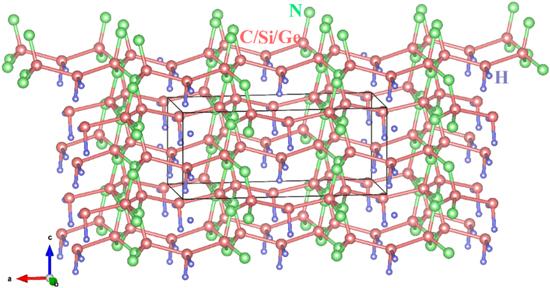
Figure 1. The crystal structures of X2 N2 (XH2 ) (X = C, Si, Ge). |
Table 1. The calculated lattice parameters (Å) of X2 N2 (XH2 ) (X = C, Si, Ge) with different functionals. |
| a | b | c | V | ||
|---|---|---|---|---|---|
| C2 N2 (CH2 ) | GGA | 8.1403 | 4.6462 | 4.1162 | 155.680 |
| LDA | 8.0061 | 4.5658 | 4.0656 | ||
| sX-LDAa | 8.1222 | 4.4630 | 4.1177 | ||
| Experimentala | 7.6250 | 4.4900 | 4.0470 | ||
| Si2 N2 (SiH2 ) | GGA | 11.6131 | 5.5769 | 4.9216 | 318.748 |
| LDA | 11.3936 | 5.4473 | 4.8228 | 299.326 | |
| Ge2 N2 (GeH2 ) | GGA | 12.1665 | 5.9103 | 5.2103 | 374.663 |
| LDA | 11.7703 | 5.6907 | 5.0368 | 337.376 |
Reference [17]. |
3.2. Stability
Table 2. The elastic constants (GPa) and elastic modulus (GPa) of X2 N2 (XH2 ) (X = C, Si, Ge). |
| C 11 | C 22 | C 33 | C 44 | C 55 | C 66 | C 12 | C 13 | C 23 | B | G | E | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C2 N2 (CH2 ) | GGA | 471 | 464 | 712 | 275 | 186 | 150 | 53 | 116 | 104 | 237 | 208 | 483 |
| GGAa | 505 | 489 | 728 | 283 | 186 | 143 | 85 | 122 | 99 | 256 | 210 | 494 | |
| LDA | 554 | 547 | 794 | 308 | 212 | 170 | 104 | 137 | 120 | 286 | 234 | 552 | |
| LDAa | 529 | 510 | 799 | 309 | 201 | 145 | 77 | 142 | 119 | 272 | 222 | 524 | |
| Si2 N2 (SiH2 ) | GGA | 199 | 200 | 240 | 104 | 50 | 43 | 58 | 45 | 48 | 104 | 68 | 167 |
| LDA | 218 | 176 | 257 | 114 | 51 | 42 | 51 | 63 | 59 | 109 | 69 | 171 | |
| Ge2 N2 (GeH2 ) | GGA | 137 | 146 | 182 | 74 | 37 | 36 | 44 | 34 | 33 | 76 | 51 | 125 |
| LDA | 198 | 114 | 152 | 88 | 34 | 57 | 39 | 25 | 13 | 67 | 58 | 135 |
Reference [21]. |
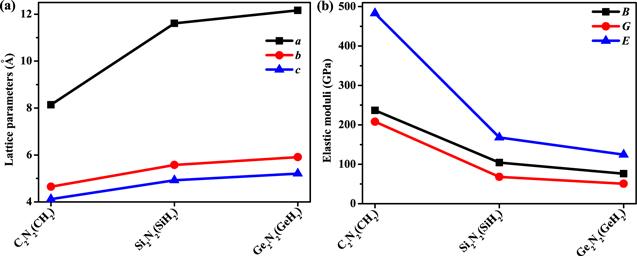
Figure 2. The lattice parameters (a) and elastic modulus (b) of X2 N2 (XH2 ) (X = C, Si, Ge), respectively. |
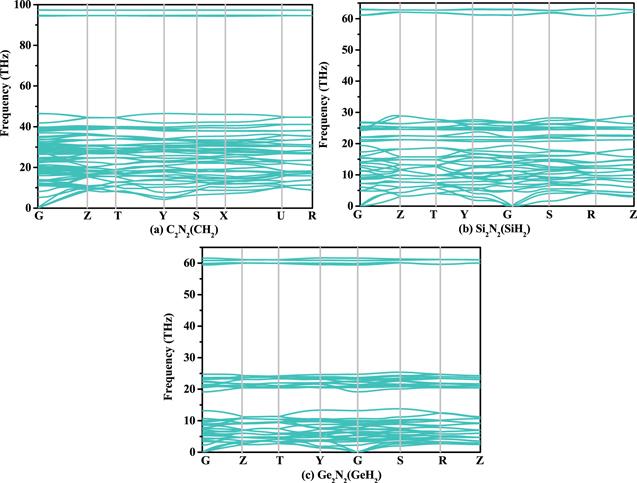
Figure 3. The phonon spectra of C2 N2 (CH2 ) (a), Si2 N2 (SiH2 ) (b) and Ge2 N2 (GeH2 ) (c), respectively. |
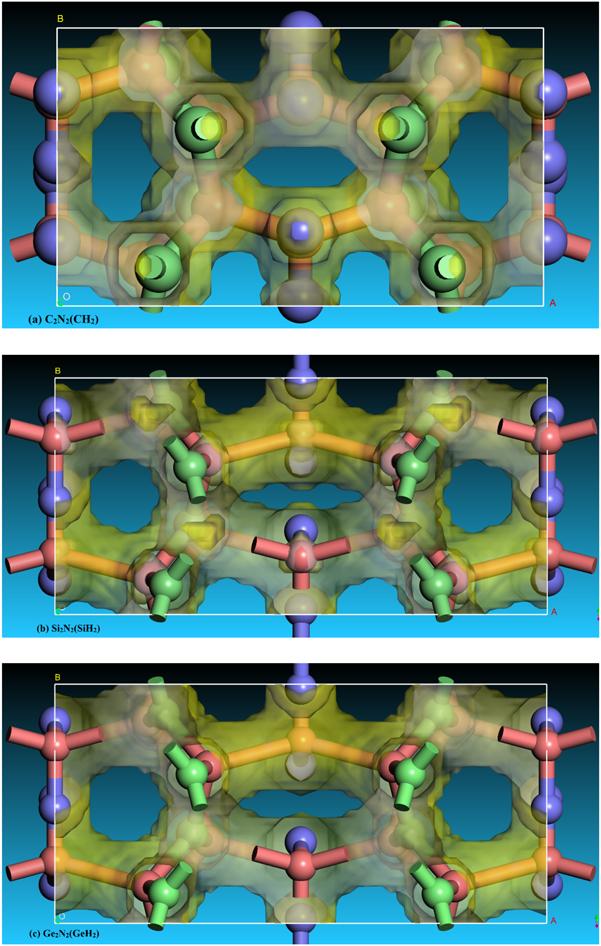
Figure 4. The electron localization function (ELF) distribution of C2 N2 (CH2 ), Si2 N2 (SiH2 ) and Ge2 N2 (GeH2 ) with an isovalue of 0.75. |
3.3. Electronic properties
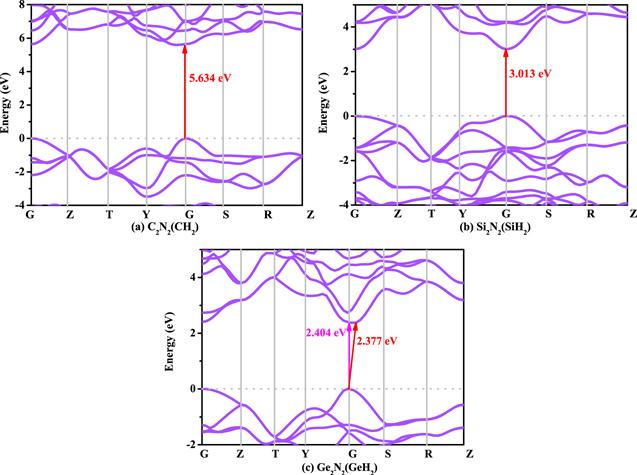
Figure 5. The electronic band structures of C2 N2 (CH2 ) (a), Si2 N2 (SiH2 ) (b), and Ge2 N2 (GeH2 ) (c), respectively. |
3.4. Debye temperature
Table 3. The calculated density (g cm−3 ), the longitudinal, transverse and mean wave velocity (v s , v p , v m in m s−1 ), and the Debye temperature (K) for X2 N2 (XH2 ) (X = C, Si, Ge). |
| ρ | v p | v s | v m | ΘD | |
|---|---|---|---|---|---|
| C2 N2 (CH2 ) | 2.8186 | 13 508 | 8590 | 9445 | 1589 |
| Si2 N2 (SiH2 ) | 2.3815 | 9041 | 5344 | 5920 | 849 |
| Ge2 N2 (GeH2 ) | 4.3931 | 5725 | 3407 | 3772 | 474 |
3.5. Mechanical anisotropy properties
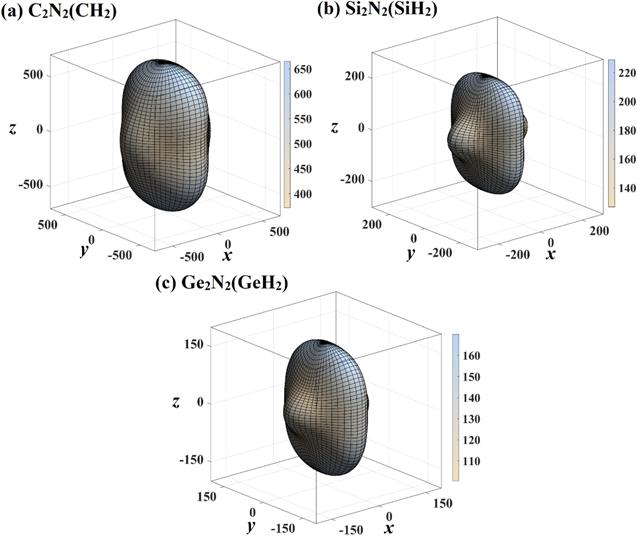
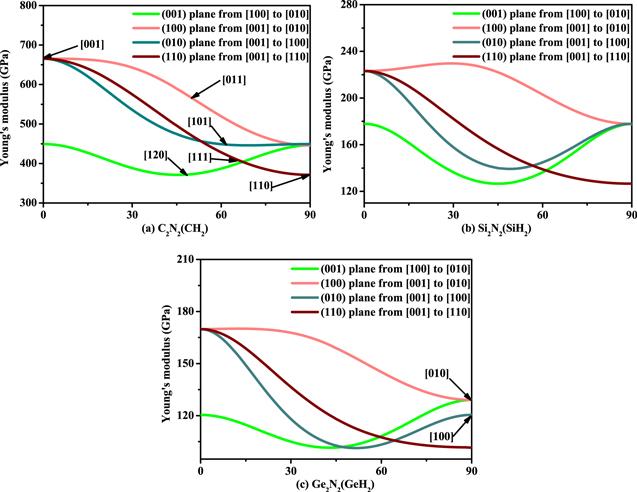
Figure 6. The directional dependence of Young’s modulus of C2 N2 (CH2 ) (a), Si2 N2 (SiH2 ) (b), and Ge2 N2 (GeH2 ) (c), respectively. |
Table 4. The calculated the maximum values, the minimum values, and ratio of Young’s modulus, shear modulus and Poisson’s ratio of X2 N2 (XH2 ) (X = C, Si, Ge). |
| E | G | v | |||||||
|---|---|---|---|---|---|---|---|---|---|
| E max | E min | Ratio | G max | G min | Ratio | v max | v min | Ratio | |
| C2 N2 (CH2 ) | 665 | 372 | 1.79 | 275 | 150 | 1.83 | 0.29 | 0.07 | 4.14 |
| Si2 N2 (SiH2 ) | 229 | 127 | 1.80 | 104 | 43 | 2.42 | 0.48 | 0.07 | 6.86 |
| Ge2 N2 (GeH2 ) | 170 | 101 | 1.68 | 74 | 36 | 2.05 | 0.42 | 0.07 | 6.00 |
Table 5. The calculated the maximum values, the minimum values, and ratio of Young’s modulus, shear modulus and Poisson’s ratio of X2 N2 (XH2 ) (X = C, Si, Ge) in (100) plane, (010) plane, and (001) plane. |
| (100) plane | (010) plane | (001) plane | |||||||
|---|---|---|---|---|---|---|---|---|---|
| E max | E min | Ratio | E max | E min | Ratio | E max | E min | Ratio | |
| C2 N2 (CH2 ) | 665 | 446 | 1.49 | 665 | 446 | 1.49 | 449 | 372 | 1.21 |
| Si2 N2 (SiH2 ) | 229 | 178 | 1.29 | 223 | 140 | 1.59 | 178 | 127 | 1.40 |
| Ge2 N2 (GeH2 ) | 170 | 129 | 1.32 | 170 | 101 | 1.68 | 129 | 102 | 1.27 |
| | |||||||||
| (100) plane | (010) plane | (001) plane | |||||||
| | |||||||||
| G max | G min | Ratio | G max | G min | Ratio | G max | G min | Ratio | |
| C2 N2 (CH2 ) | 275 | 150 | 1.83 | 275 | 150 | 1.83 | 275 | 150 | 1.83 |
| Si2 N2 (SiH2 ) | 104 | 43 | 2.42 | 104 | 43 | 2.42 | 104 | 43 | 2.42 |
| Ge2 N2 (GeH2 ) | 74 | 36 | 2.06 | 74 | 36 | 2.06 | 74 | 36 | 2.06 |
| | |||||||||
| (100) plane | (010) plane | (001) plane | |||||||
| | |||||||||
| v max | v min | Ratio | v max | v min | Ratio | v max | v min | Ratio | |
| C2 N2 (CH2 ) | 0.22 | 0.07 | 3.14 | 0.28 | 0.08 | 3.50 | 0.24 | 0.08 | 3.00 |
| Si2 N2 (SiH2 ) | 0.26 | 0.07 | 3.71 | 0.40 | 0.19 | 2.11 | 0.47 | 0.10 | 4.70 |
| Ge2 N2 (GeH2 ) | 0.29 | 0.07 | 4.14 | 0.39 | 0.14 | 2.76 | 0.41 | 0.11 | 3.73 |
Figure 7. Orientation dependence of Young’s modulus of C2 N2 (CH2 ) (a), Si2 N2 (SiH2 ) (b), and Ge2 N2 (GeH2 ) (c), respectively. |
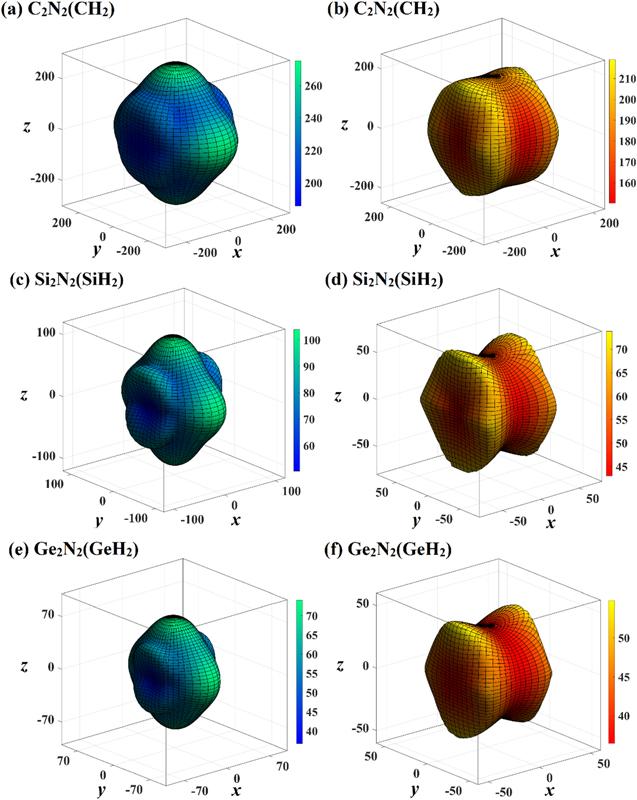
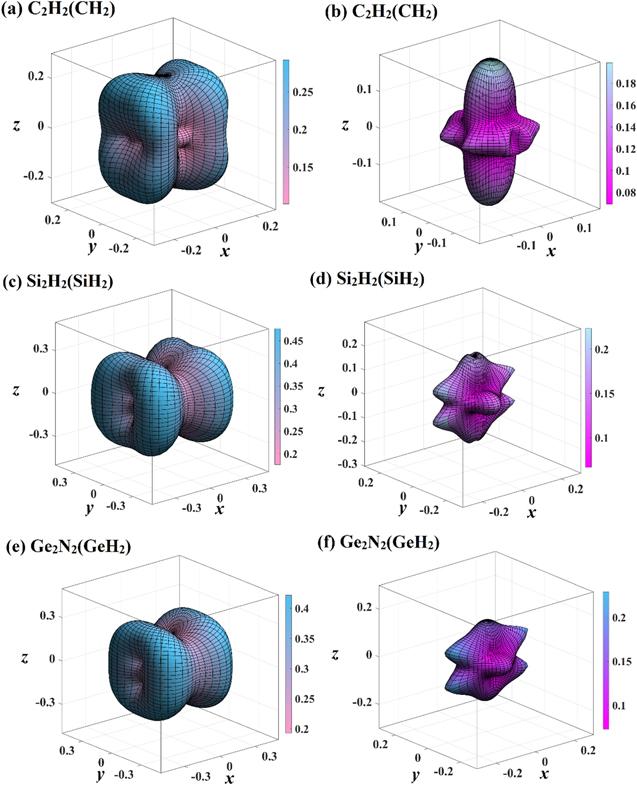
Figure 8. The directional dependence of shear modulus of C2 N2 (CH2 ) (a), (b), Si2 N2 (SiH2 ) (c), (d), and Ge2 N2 (GeH2 ) (e), (f), respectively. The blue green three-dimensional surface constructions represent the maximum values of shear modulus, and the red yellow surface constructions represent the minimum values of shear modulus, respectively. |
Figure 9. The directional dependence of Poisson’s ratio of C2 N2 (CH2 ) (a), (b), Si2 N2 (SiH2 ) (c), (d), and Ge2 N2 (GeH2 ) (e), (f), respectively. The light blue pink three-dimensional surface constructions represent the maximum values of Poisson’s ratio, and the light blue purple surface constructions represent the minimum values of Poisson’s ratio, respectively. |