1. Introduction
1.1. Ring-like patterns in experiments
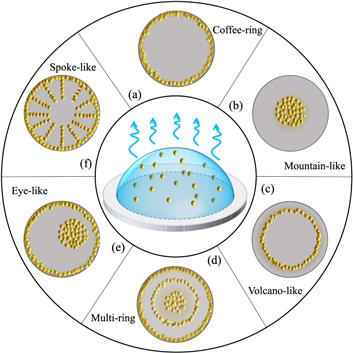
Figure 1. Schematic diagram of experimentally observed deposition patterns of drying droplets: (a) coffee-ring; (b) mountain-like; (c) volcano-like; (d) multi-ring; (e) eye-like; (f) spoke-like, which are the density distribution of solutes left on the substrate after droplet drying. |
Figure 2. Examples of multi-ring patterns through the evaporation of droplets. (a) Typical DNA stain patterns with multi-ring formation. Reprinted figure with permission from [15]. Copyright (2020) the American Physical Society. (b) MEH-PPV ring patterns in a sphere-on-flat configuration. Reprinted figure with permission from [14]. Copyright (2020) the American Physical Society. |
1.2. Conventional hydrodynamic theory of drying droplets
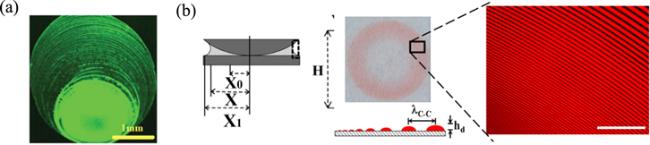
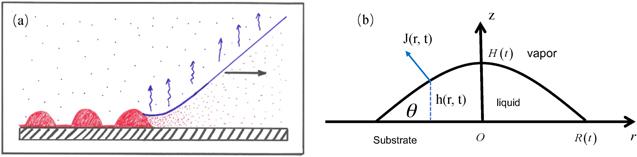
Figure 3. (a) Sketch of the essential core part of the geometry of every deposition process where material is left behind by a moving CL of suspension or solution with a volatile solvent. Reprinted from publication [27]. Copyright (2020), with permission from Elsevier. (b) Schematic of a droplet with axis-symmetry in a cylindrical coordinate system (side view). Relevant parameters are the radius of the CL R(t), height of the droplet at the center H(t), contact angle θ(t), evaporation rate J(r, t) and profile of liquid-vapor interface h(r, t). |
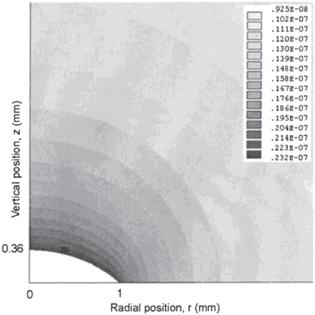
Figure 4. Contour plot of the vapor concentration distribution above a droplet of radius R = 1 mm and height h0 = 0.364 mm. Parameters used in the FEM method are vapor diffusivity D = 26.1 mm2/s, relative humidity H = 0.40 and saturated vapor concentration on the droplet surface cv = 2.32 × 10−8 g/mm3. Gray bars represent the vapor concentration in g/mm3. Reprinted with permission from [31]. Copyright (2020) the American Chemical Society. |
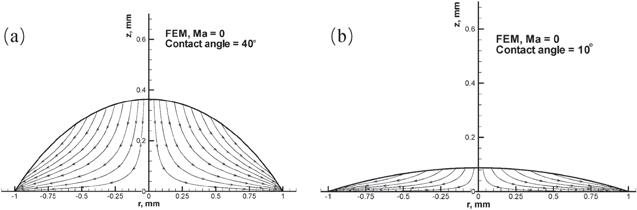
Figure 5. Streamline plots of the evaporation-induced fluid flow inside the droplets from the FEM for droplets with a contact angle of (a) 40° and (b) 10°. Reprinted with permission from [32]. Copyright (2020) the American Chemical Society. |
2. OVP theory of drying droplets
2.1. Shape evolution equations of evaporating droplets
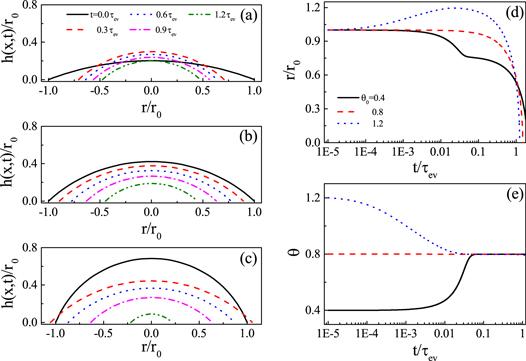
Figure 6. Droplet shape evolution for evaporation on frictionless substrate for three situations: (a) θ0 < θe, (b) θ0 = θe and (c) θ0 > θe. Corresponding evolution of the CL r/r0 is shown in panel (d) and the contact angle θ is shown in panel (e). For all calculations, θe = 0.8 and kev = 0.001. Reproduced with permission from [65]. |
2.2. Deposition patterns
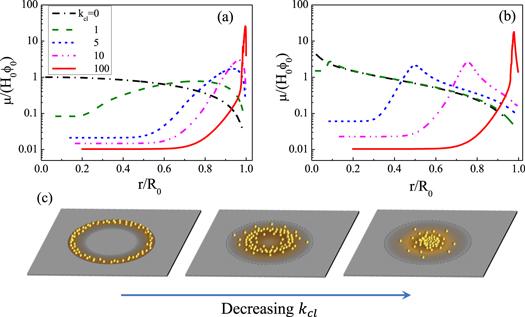
Figure 7. (a) and (b) are the profile of the deposits left on the substrate when the drying is completed. Transition from coffee-ring to volcano-like and then mountain-like pattern induced by changing the value of kcl from 100 to 0. (a) The case of a fast evaporation rate characterized by kev = 1 and (b) the case where the evaporation rate is small with kev = 10−3. For both cases, θe = θ0 = 0.2 and Δt/τev = 10−5. (c) Sketch map of the transition from coffee-ring to mountain-like pattern. Reprinted figure with permission from [64]. Copyright (2020) the American Physical Society. |
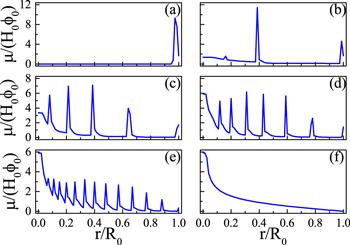
Figure 8. Various multi-ring deposition patterns of drying droplets with different values of θR: (a) 0.00, (b) 0.08, (c) 0.16, (d)0.24, (e) 0.32 and (f) 0.4. The larger θR is, the higher the moving ability of the CL. For all calculations, θ0 = θe = 0.4, R0 = 4 and kev = 10−3. Reprinted with permission from [67]. Copyright (2020) the American Chemical Society. |
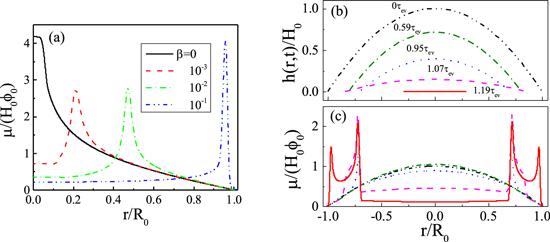
Figure 9. (a) shows the effect of a surfactant in the deposition patterns. When the initial surfactant concentration β is increased, the deposition pattern changes from mountain-like to volcano-like and then to coffee-ring pattern. All parameters are kcl = 0, kev = 0.002 and α = 1.0. (b) and (c) are the formation of the two-ring deposition pattern; (b) is the evolution of the profile of the surface and (c) is the evolution of the density distribution of solutes left on the substrate, μ(r, t)/(H0φ0). For (b) and (c), kev = 10−4, kcl = 0, α = 0.3 and β = 0.1. Reprinted with permission from [68]. Copyright (2020) the American Chemical Society. |
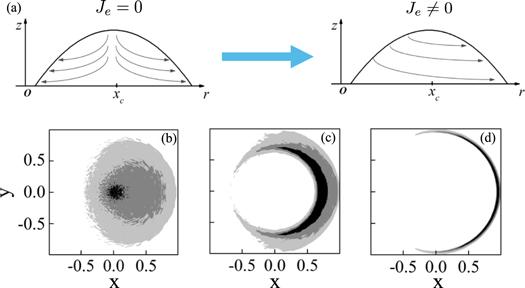
Figure 10. (a) is the sketch map of the influence of the existence of asymmetric evaporation rate Je in the evaporation-induced fluid flow inside the droplet. (b), (c) and (d) are the asymmetric deposition patterns calculated by the MC simulation. (b) Fan-like deposition is obtained when kcl = 0 and an asymmetric volcano-like deposition pattern is obtained when kcl = 10. (c) Eclipse-like deposition pattern is obtained when kcl = 50. For all the calculations θe = θ0 = 0.2, Δt/τev = 10−5, kev = 0.001 and Je = 0.3. Adapted with permission from [69]. Copyright (2020) the American Chemical Society. |
3. Perspectives and conclusion
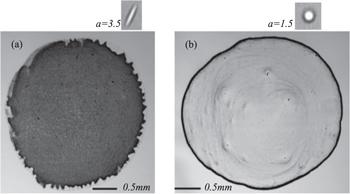
Figure 11. Images of the final distributions of ellipsoids (a) and spheres (b) after evaporation. Coffee-ring pattern changes to a uniform thin film by replacing the spherical solutes with ellipsoidal solutes, while all other experimental conditions remain the same. Reprinted by permission from [72], Copyright (2020). |