1. Introduction
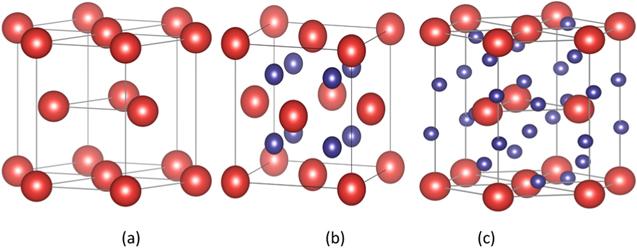
Figure 1. Schematic representation of the different erbium hydride configurations (a) Er, (b) ErT2, (c) ErT3. |
2. Simulation method
3. Results and discussion
3.1. Lattice constants
Table 1. The calculated lattice constants (Å) of three erbium hydrides. |
| α-Er | β-ErH2 | γ-ErH3 | |
|---|---|---|---|
| This work | a 3.575 | a b c 5.125 | a 6.272 |
| c 5.556 | c 6.526 | ||
| References | a 3.559 | a b c 5.123 | a 6.235 |
| c 5.587 | c 6.496 |
3.2. Elastic constants
Table 2. Calculated and reference values (Gpa) of elastic constants. |
| α-Er | β-ErH2 | γ-ErH3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No He | He-tetra | He-octa | No He | He-tetra | He-octa | No He | He-tetra | He-octa | |
| C11 | 83.53 | 78.14 | 78.80 | 145.48 | 117.02 | 129.29 | 196.33 | 186.74 | 180.03 |
| (86.15) | (142.3) | ||||||||
| C12 | 24.06 | 24.36 | 24.04 | 61.09 | 73.60 | 64.51 | 56.59 | 63.39 | 57.56 |
| (23.86) | (64.1) | ||||||||
| C13 | 25.51 (28.37) | 25.88 | 25.42 | 21.27 | 23.26 | 26.81 | |||
| C14 | 1.34 | 0.45 | 1.12 | ||||||
| C33 | 87.78 | 85.19 | 85.49 | 171.99 | 169.32 | 172.45 | |||
| (91.28) | |||||||||
| C44 | 27.92 | 27.69 | 27.94 | 74.37 | 66.20 | 67.83 | 41.79 | 33.41 | 32.41 |
| (24.13) | (75.0) | ||||||||
3.3. Elastic modulus
Table 3. Calculated values (Gpa) for the elastic modulus. |
| α-Er | β-ErH2 | γ-ErH3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No He | He-tetra | He-octa | No He | He-tetra | He-octa | No He | He-tetra | He-octa | |
| Y | 70.77 | 67.27 | 68.00 | 144.07 | 142.65 | 134.14 | 140.93 | 125.72 | 104.64 |
| G | 29.07 | 27.47 | 27.86 | 59.24 | 58.35 | 54.32 | 59.12 | 51.18 | 43.49 |
| B | 41.67 | 40.71 | 40.56 | 84.53 | 84.29 | 84.26 | 76.23 | 77.14 | 58.70 |
| B/G | 1.43 | 1.48 | 1.46 | 1.43 | 1.44 | 1.55 | 1.28 | 1.50 | 1.34 |
3.4. Electronic structural analysis
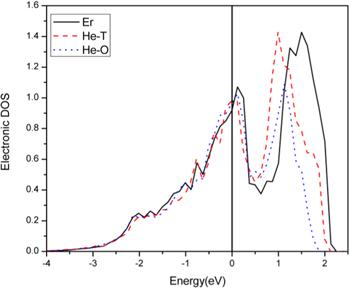
3.4.1. The effect of He on α-Er
Figure 2. Density of states diagram of the d orbital of an Er atom. |
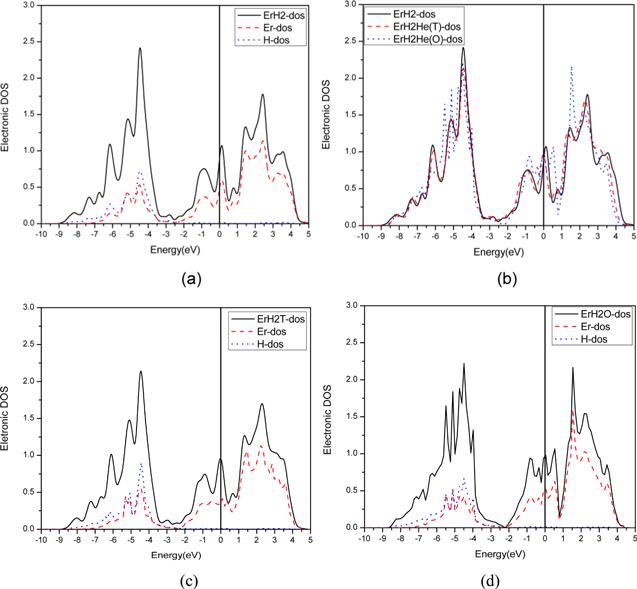
3.4.2. The effect of He on β-ErH2
Figure 3. Density of states diagram of ErH2. (a) The total density of states (TDOS) of ErH2 and the (density of states (DOS) of the d orbital of Er and the s orbital of H, (b) TDOS of pure ErH2, ErH2 with He in the octahedral site, and the tetrahedral site. (c) TDOS of ErH2 with He in the T site and the DOS of the d orbital of Er and the s orbital of H. (c) TDOS of ErH2 with He in the O site and the DOS of the d orbital of Er and the s orbital of H. |
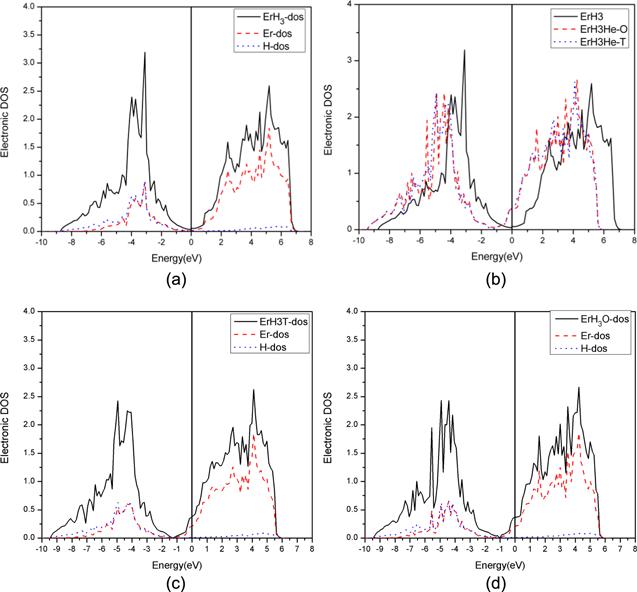
3.4.3. The effect of He on γ-ErH3
Figure 4. Density of states diagram of ErH3 (a) TDOS of ErH3 and the DOS of the d orbital of Er and the s orbital of H, (b) TDOS of pure ErH3, ErH3 with He in the octahedral site and the tetrahedral site. (c) TDOS of ErH3 with He in the T site and the DOS of the d orbital of Er and the s orbital of H. (c) TDOS of ErH3 with He in the O site and the DOS of the d orbital of Er and the s orbital of H. |
4. Conclusions
| (1) The mechanical properties of all three erbium hydrides decreased due to the production of He. He has the biggest impact on the mechanical properties of ErH3 and the decrease of the elastic modulus (a mechanical property of ErH3) can be more than 20%. | |
| (2) The calculated results for the densities of states were consistent with those of the mechanical parameters. The results for the densities of states showed that the destruction of the Er–H bond and the establishment of an Er–He bond led to a reduction in the mechanical properties. | |
| (3) Research also shows that the materials were still ductile and that no helium embrittlement appeared under the conditions described in this work. This indicates that helium embrittlement takes place at a specific He density in erbium hydrides. |