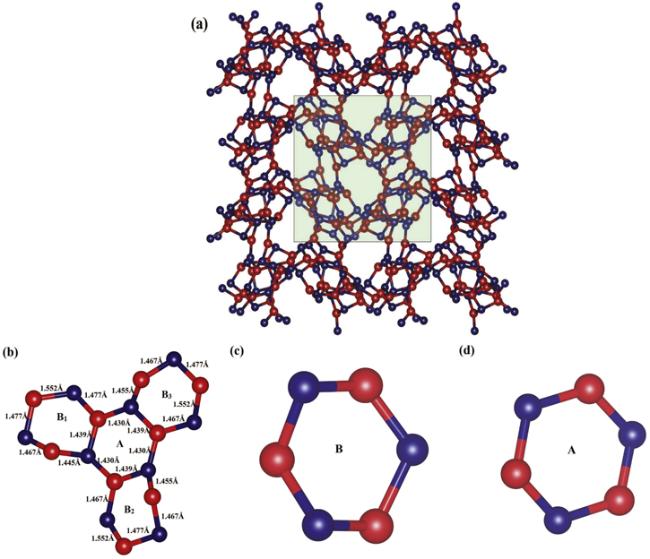
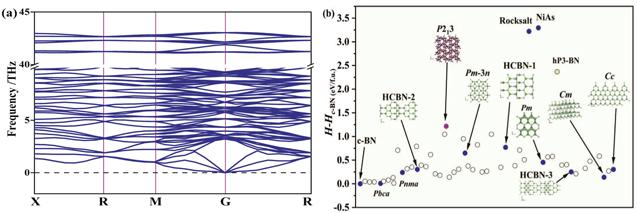
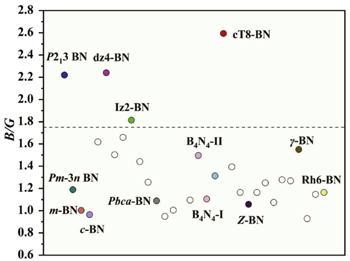
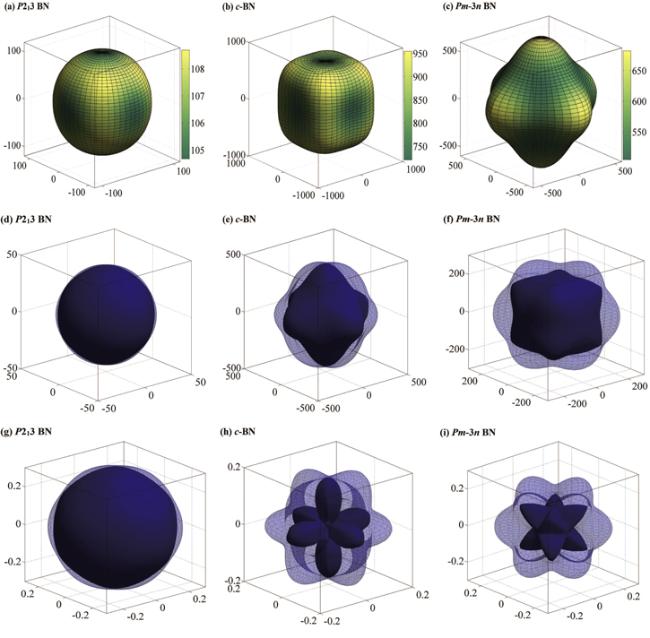
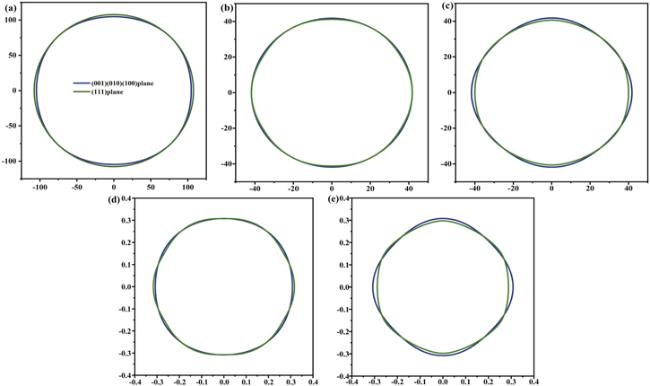
According to the elastic modulus and the density of the material, the Debye temperature can be expressed as follows: ${{\rm{\Theta }}}_{D}={v}_{m}(h/{k}_{B}){[3n/(4\pi )({N}_{A}\rho /M)]}^{1/3}$, [
57] where
h is Planck's constant,
kB is Boltzmann's constant,
NA is Avogadro's number,
n is the number of atoms in the molecule,
M is the molecular weight, and
ρ is the density. The average sound velocity
vm can be calculated from ${v}_{m}\,={[(2/{v}_{l}^{3}+1/{v}_{t}^{3})/3]}^{-1/3}$, where the transverse wave velocity
vt and longitudinal wave velocity
vl are estimated through Navier' equations:
vt = (
G/
ρ)
1/2,
vl = [(
B + 4
G/3)/
ρ]
1/2 [
58]. In the main direction, the sound velocity of the tetragonal symmetry is given by the following expression: In the [111] propagation direction, the longitudinal wave velocity
vl in the [111] polarization direction is calculated by ${[({C}_{11}+2{C}_{12}+4{C}_{44})/\rho ]}^{1/2}$, and the transverse wave velocity
vt in the [11-2] polarization direction is calculated by ${[({C}_{11}-{C}_{12}+{C}_{44})/3\rho ]}^{1/2}$. At the same time, in the [110] propagation direction, the longitudinal wave velocity
vl in the [110] polarization direction is calculated by ${[({C}_{11}+{C}_{12}+2{C}_{44})/2\rho ]}^{1/2}$, and the transverse wave velocity
vt in the [1-10] polarization direction is calculated by ${[({C}_{11}-{C}_{12})/\rho ]}^{1/2}$. Moreover, in the [100] propagation direction, the longitudinal wave velocity
vl in the [110] polarization direction is calculated by ${({C}_{11}/\rho )}^{1/2}$, the transverse wave velocity
vt in the [010] polarization direction is calculated by ${({C}_{44}/\rho )}^{1/2}$, and the transverse wave velocity
vt2 in the [001] polarization direction is calculated by ${({C}_{12}/\rho )}^{1/2}$. The related results are presented in table
3. The [111], [110] and [100] directions in the first column of table
3 are the propagation directions, and the second column is the polarization direction. As is presented in table
3, the Debye temperature of
P2
13 BN is 684 K, which is smaller than that of
Pm-3
n BN and
c-BN, and the average sound velocity, transverse wave velocity, and longitudinal wave velocity of
P2
13 BN are also smaller than those of
Pm-3
n BN and
c-BN. The study of the sound velocity has revealed that it is also anisotropic. The largest value of the sound velocity for
P2
13 BN is 14 174 m/s in the [111]
vl direction, the same direction as
Pm-3
n BN and
c-BN, while the value of
P2
13 BN is about three fifths that of
Pm-3
n BN and half of
c-BN. The smallest value of
P2
13 BN is 4309 m s
−1 in the [11-2]
vt12 direction, different to the direction of
Pm-3
n BN and
c-BN. The value of
vl in the [110] direction and the value of
vt1 in the [1-10] direction for
Pm-3
n BN and
c-BN are about twice those of
P2
13 BN. Meanwhile, the value of sound velocity in the [010]
vt1 direction is the same as that in the [001]
vt2 direction. As for
Pm-3
n BN, the values of
vt1 in [1-10] and
vl in [100] even exceed the values of
c-BN.