1. Introduction
Figure 1. Chemical structure of 1-butyl-3-methylimidazolium chloride. |
2. Dual ionic and organic nature of ILs
Figure 2. (a) Cage energy landscape characterized by curvature, slope, and depth, corresponding to force constant, force, and activation energy experienced by molecules, respectively. (b) Schematic illustration of cage structures and cage energy landscape in inorganic salts, ionic liquids, and organic solvents. The cage energy landscape of inorganic salts is deep and steep, whereas that of ionic liquids is still deep but much more gently. Organic solvents and ionic liquids have a similar slope and curvature near the minimum of the cage energy landscape, but the depths for the organic solvents are much lower. Reprinted with permission from [85]. |
3. Nanoscale segregation liquid (NSL) phase
Figure 3. (a) Molecular structure and coarse-graining scheme for the simulated IL. (b) Snapshot illustrating the NSL phase. The white spheres represent the cationic terminal groups, the gold spheres represent the cationic head groups, and the red spheres represent the anions. The ellipses in blue indicate the approximate positions of the nonpolar tail domains. Reprinted with permission from [104]. Copyright 2007 American Chemical Society. |
4. ILC phases
Figure 4. (a) HOP for cationic head groups, anions, and cationic tail groups. (b) OCF for side chains. (c) Molecular structure of the investigated IL system and the corresponding coarse-grained model. Reprinted with permission from [81]. Copyright 2013 American Chemical Society. |
Figure 5. Percolation phase transition. (a)–(c) Several largest clusters in C12, C16 and C22 systems, respectively, with the largest cluster colored blue. (a) The largest cluster in C12 is very small and not well aligned. (b) The largest cluster in C16 is larger and better aligned in parallel, but still local with little orientation correlation between clusters. (c) The largest cluster in C22 almost fills in the whole simulation box, indicating that the majority of the side chains are globally aligned in parallel and well connected. (d) Normalized sizes of the largest and second-largest clusters for all systems. (e) Average cluster size versus side-chain length. After the percolation phase transition, the largest cluster is not counted in the calculation of the average cluster size. (f) Correlation length versus side-chain length. For a finite system, both the average cluster size and the correlation length reach their maxima at the phase transition point. The correlation length is directly related to the average cluster size. Reprinted with permission from [116]. |
5. Solid–solid and melting phase transitions
Figure 6. HOPs for some CG sites as a function of temperature. The error bars are almost invisible since they are smaller than the size of the markers. Cr represents the crystal phase, SmA the smectic A phase, and Iso the NSL phase. Reprinted with permission from [119]. Copyright 2015 American Chemical Society. |
Figure 7. (a) HOP values for some CG sites as a function of time during the transition from the NSL phase into the smectic A phase, along with snapshots taken at T = 505 K after simulated for 0, 48, and 72 ns. (b) HOP values for some CG sites as a function of time during the transition from the smectic A phase into the crystal phase, along with snapshots taken at T = 480 K after simulated for 0, 5, and 20 ns. Reprinted with permission from [119]. Copyright 2015 American Chemical Society. |
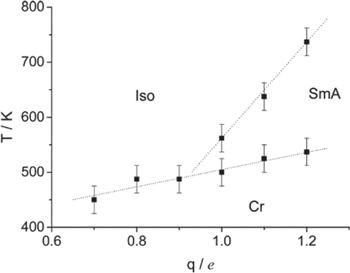
Figure 8. Phase diagram of the IL system simulated with the EF-CG model. Cr represents the crystal phase, SmA the smectic A phase, and Iso the NSL phase. Reprinted with permission from [120]. Copyright 2016 American Chemical Society. |
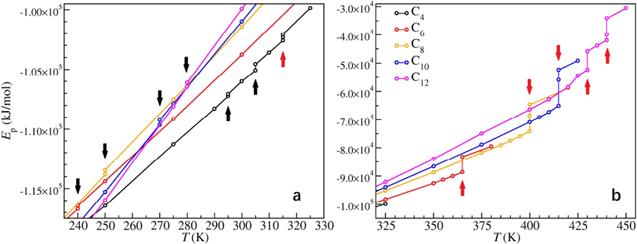
Figure 9. Caloric curves during heating in [CnMIm][NO3] systems. (a) From T = 240 K to 325 K. (b) From T = 325 K to 450 K. The solid–solid phase transition points are marked by black arrows, and the melting transition points are marked by red arrows. Reprinted with permission from [84]. Copyright 2018 American Chemical Society. |
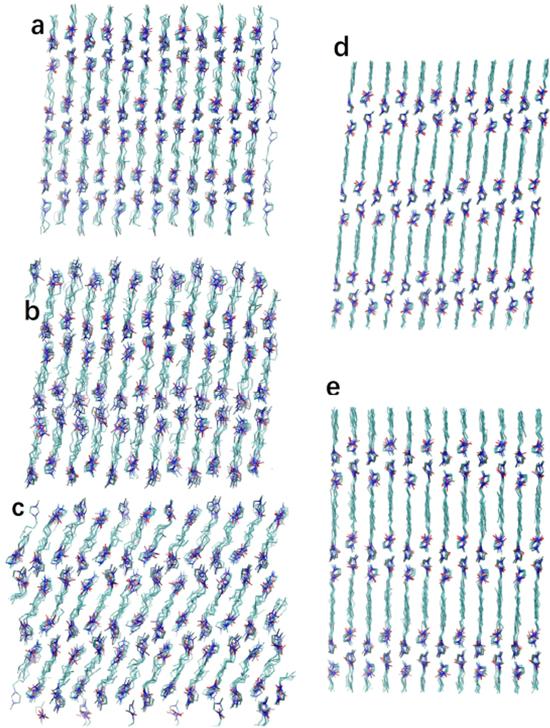
Figure 10. Snapshots of the structures before and after the solid–solid phase transitions. (a) C4 at 295 K before the phase transition. (b) C4 at 300 K after the first but before the second phase transition. (c) C4 at 305 K after the phase transition. (d) C8 at 250 K before the phase transition. (e) C8 at 250 K after the phase transition. All these snapshots are taken from the [100] direction. Reprinted with permission from [84]. Copyright 2018 American Chemical Society. |
6. Partially arrested glassy state
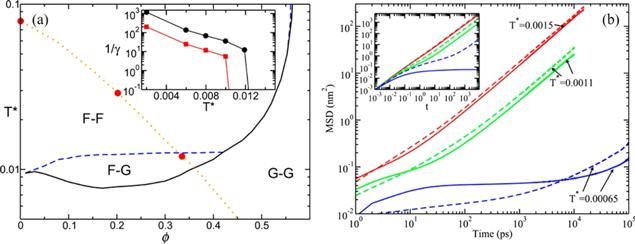
Figure 11. (a) Arrest lines predicted by the SCGLE theory for the primitive model with a size asymmetry adequate for the simulated IL (1:3.5). The fluid region is labelled as F–F. The G–G region corresponds to fully arrested states. The partially arrested phenomenon occurs in the F–G region. The inset shows the values of the parameter 1/γα following the isobaric trajectory with a pressure of 1 atm indicated by the dotted line. Circles correspond to anions (smaller particles) and squares to cations (larger particles). (b) MSDs for [EMIM][BF4]. Dashed lines represent cations and solid lines represent anions. The inset shows the theoretical calculation results of the MSDs over the isobaric trajectory (big circles in the left panel). The first two temperatures are located in the F–F region and the last one inside the F–G region. Solid and dashed lines correspond to anions and cations, respectively. Reprinted from [83] with permission from AIP Publishing. |
7. Liquid–liquid phase separation of IL/benzene mixture
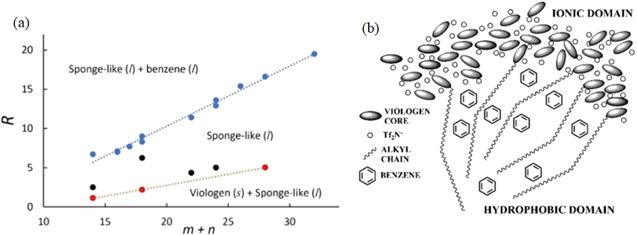
Figure 12. (a) Phase diagram of the mole ratio R = Nbenzene/Nviologen versus m + n, the total number of carbon groups in the cationic alkyl chains, measured by experiment. The blue symbols represent the coexistence line between liquid benzene and sponge-like liquid phase, the red symbols represent the coexistence line between solid viologen salt and sponge-like liquid phase, and the black symbols represent intermediate state points with only the sponge-like liquid phase present. (b) Schematic representation of the sponge-like phase. The typical nanoscale segregation normally observed in ionic liquids is reproduced in the sponge-like phase after benzene molecules are absorbed in the hydrophobic regions. For the sake of clarity, only a few alkyl chains are schematically drawn. Reprinted with permission from [135]. Copyright 2020 American Chemical Society. |