1. Introduction
2. The model-related assumptions
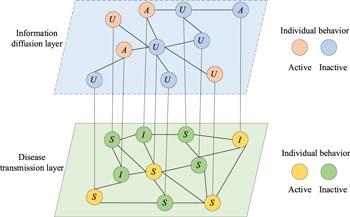
Network structure assumption. As shown in figure 1, our model is implemented on a multiplex network. To illustrate it, we construct a two-layer network, which is used to describe the diffusion of disease-related information on the social networks layer and the transmission of diseases in the physical contact layer, respectively. For simplicity, we assume that the multiplex network is unweighted and undirected. The relationship between the two layers is a coupling dynamic process of disease transmission and information diffusion. A virtual connection between two-layer networks means that the mapping relationship of node pairs, and the individuals in the two-layer networks are represented by circles. The upper layer stands for the disease-related information diffusion on social networks (e.g., Twitter, Facebook and WeChat) denoted by the $\mathrm{UAU}$ (unaware-aware-unaware) layer. The nodes are divided into two states: unaware (${\rm{U}}$) and (${\rm{A}}$) aware. State ${\rm{U}}$ indicates that the individuals are not aware of disease-related information; state ${\rm{A}}$ indicates that individuals are aware of the disease-related information. It is worth noting that, on the one hand, individuals who are not aware of the existence of the disease will not take any measures to avoid being infected by the disease; on the other hand, individuals who are aware of the existence of the disease will diffuse disease-related information to their neighbors and take measures to avoid being infected by the disease. Among them, if an unaware individual contacts an aware neighbor, he or she will acquire the disease-related information with a probability $\lambda .$ Furthermore, an aware individual will forget the disease-related information with the probability $\delta .$
Figure 1. Schematic diagram of the coupled $\mathrm{UAU}\unicode{x02013}\mathrm{SIS}$ model multiple networks. The upper layer is the virtual information layer, where nodes have two possible states: aware ($A$) and unaware ($U$); The lower layer network denotes the physical contact layer, where nodes also have two possible states: susceptible ($S$) and infected ($I$). For the activity behavior of nodes, the orange and blue dots represent the active and inactive status of nodes in the virtual information layer, respectively. Meanwhile, the yellow and green dots denote the active and inactive status of nodes in the physical contact layer, respectively. |
Assumption of individual disease infection rate. In particular, the mapping pattern between the corresponding nodes in the two-layer network is one-to-one, that is, an individual is assumed to appear in the social network and the physical contact network at the same time. As proposed in [37, 38], in the layer of disease transmission, the disease infection rate depends on the ‘susceptibility’ of susceptible individuals and the ‘transmission ability’ of infected individuals, which can be defined as:
Assumption of asymmetric activity. Here, heterogeneous activity is used to describe the activity of individuals. Inactive individuals do not take the initiative to contact and generate connection edges in the network, and most of them wait for active nodes to activate and generate connection edges to connect to them. The individual in the proposed model will be involved in the diffusion of information and the transmission of diseases. Individuals have different levels of activity in different environments. The activity of the information transmission layer refers to whether individuals actively diffuse disease-related information; during each time step, an active individual interacts with all its neighbors, while an inactive node can only be interacted with by its active neighbors. The activity of the disease transmission layer denotes some behavioral characteristics of individuals (for example running around during the disease and going out without masks). Individuals, whether active or not, are at risk of becoming infected once they contact with infected neighbors.
Assumption of key parameters of the model. In order to introduce the proposed model more clearly, we first make assumptions about the definition of key quantities or parameters in the model in table 1.
3. The analytical results based on mean-field method
Table 1. Definition of some key quantities or parameters in the proposed disease model. |
| Symbol | Definition |
|---|---|
| $\lambda $ | The probability that unaware individuals can communicate with aware individuals to obtain information. |
| $\delta $ | The probability that an aware individual forgets information |
| $\beta $ | The probability that the susceptible individual would transmit an infection through one contact with an infected neighbor. |
| $\mu $ | The probability that an infected individual recovering to a susceptible state |
| $\alpha $ | The activity level of individuals in the information diffusion layer |
| $\omega $ | The activity level of individuals in the disease transmission layer |
| ${\beta }^{U}$ | The probability that an unaware susceptible individual is infected by one of his infected neighbors |
| ${\beta }^{A}$ | The probability that an aware susceptible individual is infected by one of his infected neighbors |
| $\phi $ | Infection reduction factor when a susceptible individual is aware of the disease. |
| ${r}_{i}$ | The probability that an individual will not be informed |
| ${q}_{i}^{U}$ | The probability that an individual who is not aware of the existence of the disease information will not be infected |
| ${q}_{i}^{A}$ | The probability that an individual who is aware of the existence of the disease information will be infected |
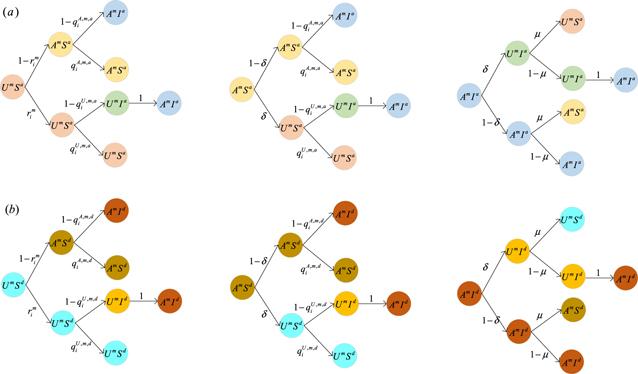
Figure 2. Transition probability trees for three active states in the information diffusion layer per time step. (a) represents the state probability tree of nodes that are active in the disease transmission layer; (b) denotes the state probability tree of nodes that are inactive in the disease transmission layer. Nodes that are active in the information diffusion layer and active in the disease transmission layer aware of disease will not be infected with probability ${q}_{i}^{A,m,a};$ nodes that are active in the information diffusion layer and inactive in the disease transmission layer aware of disease will not be infected with probability ${q}_{i}^{A,m,d};$ nodes that are active in the information diffusion layer and active in the disease transmission layer unaware of disease will not be infected with probability ${q}_{i}^{U,m,a};$ nodes that are active in the information diffusion layer and inactive in the disease transmission layer unaware of disease will not be infected with probability ${q}_{i}^{U,m,d}.$ ${r}_{i}^{m}$ is used to denote the probability that nodes that are active in the information diffusion layer will not be informed by any neighbors who are aware of the disease. $\delta $ is the probability that nodes are aware of the information of disease but may forget, and $\mu $ is the probability that infectious individuals may recover. |
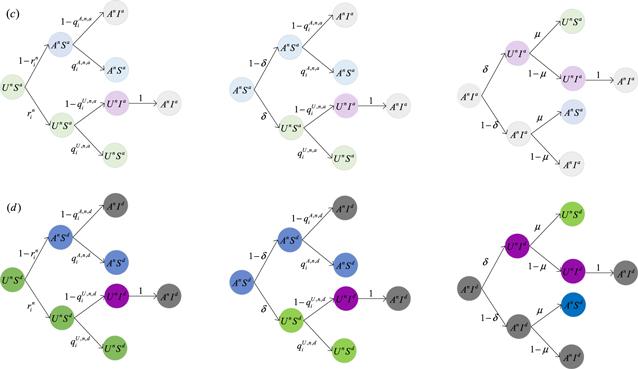
Figure 3. Transition probability trees for three inactive states in the information diffusion layer per time step. (c) represents the state probability tree of nodes that are active in the disease transmission layer; (d) denotes the state probability tree of nodes that are inactive in the disease transmission layer. Nodes that are inactive in the information diffusion layer and active in the disease transmission layer aware of disease will not be infected with probability ${q}_{i}^{A,n,a};$ nodes that are active in the information diffusion layer and inactive in the disease transmission layer aware of disease will not be infected with probability ${q}_{i}^{A,n,d};$ nodes that are inactive in the information diffusion layer and inactive in the disease transmission layer unaware of disease will not be infected with probability ${q}_{i}^{U,n,a};$ nodes that are inactive in the information diffusion layer and inactive in the disease transmission layer unaware of disease will not be infected with probability ${q}_{i}^{U,n,d}.$ ${r}_{i}^{n}$ is used to denote the probability that nodes that are inactive in the information diffusion layer will not be informed by any neighbors who are aware of the disease. |
| (1)Behavior state change in the upper layer network $\begin{eqnarray*}{U}^{m}\mathop{\longrightarrow }\limits^{1-\alpha }{U}^{n},{U}^{n}\mathop{\longrightarrow }\limits^{\alpha }{U}^{m};\end{eqnarray*}$ $\begin{eqnarray*}{A}^{m}\mathop{\longrightarrow }\limits^{1-\alpha }{A}^{n},{A}^{n}\mathop{\longrightarrow }\limits^{\alpha }{A}^{m}.\end{eqnarray*}$ | |
| (2)Behavior state change in the lower layer network $\begin{eqnarray*}{S}^{a}\mathop{\longrightarrow }\limits^{1-\omega }{S}^{d},{S}^{d}\mathop{\longrightarrow }\limits^{\omega }{S}^{a};\end{eqnarray*}$ $\begin{eqnarray*}{I}^{a}\mathop{\longrightarrow }\limits^{1-\omega }{I}^{d},{I}^{d}\mathop{\longrightarrow }\limits^{\omega }{I}^{a}.\end{eqnarray*}$ | |
| (3)Active spreading in the upper layer network $\begin{eqnarray*}{U}^{m}S+AS\mathop{\longrightarrow }\limits^{\lambda }{A}^{m}S+AS,{U}^{m}S+AI\mathop{\longrightarrow }\limits^{\lambda }{A}^{m}S+AI.\end{eqnarray*}$ | |
| (4)Inactive spreading in the upper layer network $\begin{eqnarray*}{U}^{n}S+{A}^{m}S\mathop{\longrightarrow }\limits^{\lambda }{A}^{n}S+{A}^{m}S,{U}^{n}S+{A}^{m}I\mathop{\longrightarrow }\limits^{\lambda }{A}^{n}S+{A}^{m}I.\end{eqnarray*}$ | |
| (5)Active spreading in the lower layer network $\begin{eqnarray*}\begin{array}{l}U{S}^{m}+AI\mathop{\longrightarrow }\limits^{{\beta }^{u}}U{I}^{m}+AI\mathop{\longrightarrow }\limits^{1}A{I}^{m}\\ \,+\,AI,A{S}^{m}+AI\mathop{\longrightarrow }\limits^{{\beta }^{A}}A{I}^{m}+AI.\end{array}\end{eqnarray*}$ | |
| (6)Inactive spreading in the lower layer network $\begin{eqnarray*}\begin{array}{l}U{S}^{n}+A{I}^{m}\mathop{\longrightarrow }\limits^{\omega {\beta }^{u}}U{I}^{n}+A{I}^{m}\mathop{\longrightarrow }\limits^{1}A{I}^{n}+A{I}^{m},\\ A{S}^{n}+A{I}^{m}\mathop{\longrightarrow }\limits^{\omega {\beta }^{A}}A{I}^{n}+A{I}^{m}.\end{array}\end{eqnarray*}$ | |
| (7)Recoveries |
4. The numerical simulations
Figure 4. Stationary state and critical threshold of the disease with different transmission rate $\lambda ,$ $\beta .$ Activity level $\alpha $ in the information diffusion layer is set as follows: (a) $\alpha =0.3,$ (b) $\alpha =0.6,$ (c) $\alpha =0.9,$ from left to right. Color maps represent the prevalence of the disease. The dotted line represents the threshold ${\beta }^{c},$ and the solid line represents the threshold ${\beta }^{{c}^{^{\prime} }}$ predicted by the model. Each point in grid 40 * 40 in the figure is obtained through an average of 50 numerical simulations. Dynamical parameters: information forgetting rate $\sigma =0.4,$ disease recovery rate $\mu =0.6,$ infection reduction factor $\phi =0.5,$ active level of nodes in the disease transmission layer $\omega =0.4.$ |
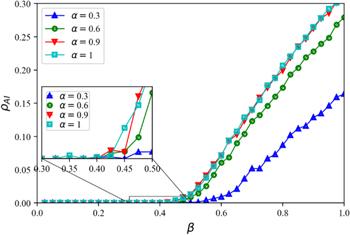
Figure 5. ${\rho }_{AI}$ as a function of the disease transmission rate $\beta $ under different activity level $\alpha $ in the information diffusion layer. Dynamical parameters: information layer spreading rate $\lambda =0.4$ and information forgetting rate $\sigma =0.4,$ disease recovery rate $\mu =0.6,$ infection reduction factor $\phi =0.5,$ active level of nodes in the disease transmission layer $\omega =0.4.$ |
Figure 6. Stationary state and critical threshold of the disease with different spreading rate $\lambda ,$ $\beta .$ Activity level $\omega $ in the disease transmission layer is set as follows: (a) $\omega =0.3,$ (b) $\omega =0.6,$ (c) $\omega =0.9,$ from left to right. Color maps represent the prevalence of the disease. The dotted line represents the threshold ${\beta }^{c},$ and the solid line represents the threshold ${\beta }^{{c}^{^{\prime} }}$ predicted by the model. Each point in grid 40 * 40 in the figure is obtained through an average of 50 numerical simulations. Dynamical parameters: information forgetting rate $\sigma =0.4,$ disease recovery rate $\mu =0.6,$ infection reduction factor $\phi =0.5,$ active level of nodes in the information diffusion layer $\alpha =0.4.$ |
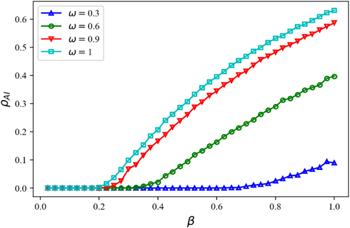
Figure 7. ${\rho }_{AI}$ as a function of the disease transmission rate $\beta $ under activity level $\omega $ in the disease transmission layer. Dynamical parameters: information diffusion rate $\lambda =0.4$ and information forgetting rate $\sigma =0.4,$ disease recovery rate $\mu =0.6,$ infection reduction factor $\phi =0.5,$ active level of nodes in the information diffusion layer $\alpha =0.4.$ |
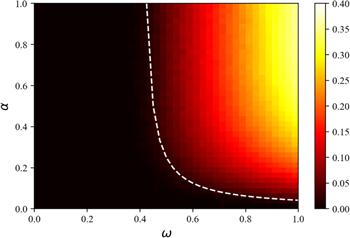
Figure 8. The heat map under the joint action of individual behavior rate $\alpha $ of the information diffusion layer and individual behavior rate $\omega $ of the disease transmission layer. The remaining parameters are set to be: information diffusion rate $\lambda =0.4$ and information forgetting rate $\sigma =0.4.$ |
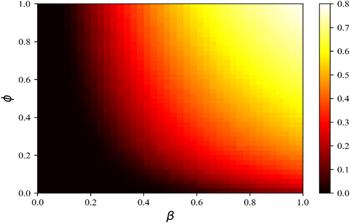
Figure 9. The heat map shows the impact $\phi $ (infection reduction factor when a susceptible individual is aware of the disease) on disease transmission. The remaining parameters are set to be: information diffusion rate $\lambda =0.4$ and information forgetting rate $\sigma =0.4,$ disease recovery rate $\mu =0.6,$ activity level on the information diffusion layer $\alpha =0.3,$ activity level on the disease transmission layer $\omega =0.3.$ |
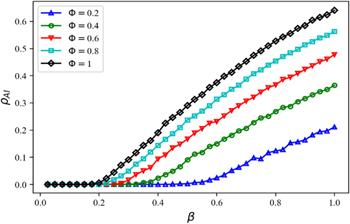
Figure 10. The final size of spreading dynamics ${\rho }^{AI}$ with different disease transmission rate $\beta $ and infection reduction factor $\phi .$ The remaining parameters are set to be: information diffusion rate $\lambda =0.4$ and information forgetting rate $\sigma =0.4,$ disease recovery rate $\mu =0.6.$ |