1. Introduction
2. Bound state solutions
3. Results and discussion
3.1. Particular cases
3.2. The bound state energy eigenvalues and lowest excitation in diatomic molecules
Table 1. Spectroscopic molecular parameters for H2, HCl, O2 diatomic molecules. |
| Molecule | μ | re | De | νe |
|---|---|---|---|---|
| (a. m. u. ) | (Å) | (cm−1) | (cm−1) | |
| H2 | 0.5041 | 0.7417 | 36 118.062 | 4395.2 |
| HCl | 0.9799 | 1.274 563 03 | 37 243 | 2990.875 |
| O2 | 8.000 | 1.207 5358 | 42 047 | 1580.194 |
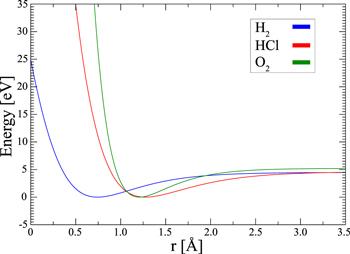
Figure 1. Potential energy curves of the diatomic molecules H2, HCl and O2 as a function of the interatomic distance. |
Table 2. The bound state energy eigenvalues ${E}_{{nl}}^{{NR}}$ of the Schrödinger molecule and ${E}_{{nl}}^{b}={E}_{{nl}}^{R}-{{Mc}}^{2}$ of the Klein–Gordon molecule in the GTHP calculated using equation ( |
| n | l | ${E}_{{nl}}^{{NR}},({{\rm{H}}}_{2})$ | ${E}_{{nl}}^{b},({{\rm{H}}}_{2})$ | ${E}_{{nl}}^{{NR}},(\mathrm{HCl})$ | ${E}_{{nl}}^{b},(\mathrm{HCl})$ | ${E}_{{nl}}^{{NR}},({{\rm{O}}}_{2})$ | ${E}_{{nl}}^{b},({{\rm{O}}}_{2})$ |
|---|---|---|---|---|---|---|---|
| (cm−1) | (cm−1) | (cm−1) | (cm−1) | (cm−1) | (cm−1) | ||
| 0 | 0 | 2163.332 479 | 2163.332 477 | 1480.444 771 | 1480.444 675 | 786.437 8165 | 786.437 6894 |
| 1 | 0 | 6327.103 462 | 6327.103 410 | 4359.482 381 | 4359.482 341 | 2338.054 436 | 2338.054 024 |
| 1 | 1 | 6444.845 686 | 6444.845 634 | 4379.847 347 | 4379.847 337 | 2340.894 357 | 2340.894 427 |
| 2 | 0 | 10 252.927 23 | 10 252.927 26 | 7125.499 716 | 7125.499 649 | 3860.922 397 | 3860.922 186 |
| 2 | 1 | 10 367.885 83 | 10 367.885 90 | 7145.313 357 | 7145.313 215 | 3863.729 451 | 3863.729 320 |
| 2 | 2 | 10 596.757 12 | 10 596.757 28 | 7184.927 896 | 7184.927 714 | 3869.343 429 | 3869.344 512 |
| 3 | 0 | 13 934.324 20 | 13 934.324 17 | 9777.517 902 | 9777.517 934 | 5354.985 484 | 5354.985 616 |
| 3 | 1 | 14 046.229 51 | 14 046.229 52 | 9796.773 872 | 9796.773 864 | 5357.759 581 | 5357.759 412 |
| 3 | 2 | 14 269.024 31 | 14 269.024 47 | 9835.273 336 | 9835.273 166 | 5363.306 888 | 5363.307 911 |
| 3 | 3 | 14 600.720 22 | 14 600.720 31 | 9892.991 022 | 9892.990 941 | 5371.629 453 | 5371.630 377 |
| 4 | 0 | 17 364.449 80 | 17 364.449 77 | 12 314.543 22 | 12 314.543 18 | 6820.187 243 | 6820.187 560 |
| 4 | 1 | 17 473.029 85 | 17 473.029 83 | 12 333.235 31 | 12 333.235 23 | 6822.927 545 | 6822.927 953 |
| 4 | 2 | 17 689.208 23 | 17 689.208 38 | 12 370.607 07 | 12 370.606 85 | 6828.408 793 | 6828.409 623 |
| 4 | 3 | 18 011.064 17 | 18 011.064 30 | 12 426.633 34 | 12 426.633 29 | 6836.630 730 | 6836.631 845 |
| 4 | 4 | 18 435.819 49 | 18 435.819 57 | 12 501.277 34 | 12 501.277 24 | 6847.592 259 | 6847.593 247 |
| 5 | 0 | 20 536.070 41 | 20 536.070 39 | 14 735.565 70 | 14 735.565 70 | 8256.471 021 | 8256.471 069 |
| 5 | 1 | 20 641.051 30 | 20 641.051 28 | 14 753.687 76 | 14 753.687 60 | 8259.177 484 | 8259.177 992 |
| 5 | 2 | 20 850.069 73 | 20 850.069 82 | 14 789.919 18 | 14 789.918 98 | 8264.591 767 | 8264.592 703 |
| 5 | 3 | 21 161.280 64 | 21 161.280 76 | 14 844.235 14 | 14 844.235 24 | 8272.713 618 | 8272.714 473 |
| 5 | 4 | 21 572.017 81 | 21 572.017 97 | 14 916.599 24 | 14 916.599 34 | 8283.541 215 | 8283.541 974 |
| 5 | 5 | 22 078.906 37 | 22 078.906 53 | 15 006.962 14 | 15 006.962 07 | 8297.076 277 | 8297.076 196 |
| 6 | 0 | 23 441.536 12 | 23 441.536 08 | 17 039.560 05 | 17 039.559 91 | 9663.779 212 | 9663.779 010 |
| 6 | 1 | 23 542.642 61 | 23 542.642 54 | 17 057.105 39 | 17 057.105 32 | 9666.452 501 | 9666.452 396 |
| 6 | 2 | 23 743.954 74 | 23 743.954 88 | 17 092.184 01 | 17 092.183 82 | 9671.798 959 | 9671.800 000 |
| 6 | 3 | 24 043.712 12 | 24 043.712 23 | 17 144.770 88 | 17 144.770 97 | 9679.820 497 | 9679.821 126 |
| 6 | 4 | 24 439.372 71 | 24 439.372 81 | 17 214.829 91 | 17 214.829 95 | 9690.513 889 | 9690.514 445 |
| 6 | 5 | 24 927.723 10 | 24 927.723 19 | 17 302.311 92 | 17 302.311 89 | 9703.880 795 | 9703.880 933 |
| 6 | 6 | 25 505.011 24 | 25 505.011 31 | 17 407.155 87 | 17 407.155 76 | 9719.918 459 | 9719.919 524 |
Table 3. The lowest rotational △E(l) and vibrational △E(n) excitation energies, all values in cm−1. |
| Molecule | H2 | H2 | HCl | HCl | O2 | O2 |
|---|---|---|---|---|---|---|
| Exc. type | l = 0 → 1 | n = 0 → 1 | l = 0 → 1 | n = 0 → 1 | l = 0 → 1 | n = 0 → 1 |
| Theorya | 117.742 224 | 4163.770 983 | 20.364 966 | 2879.037 610 | 2.839 921 | 1551.616 620 |
| Theoryb | 117.742 224 | 4163.770 933 | 20.364 996 | 2879.037 666 | 2.840 403 | 1551.616 335 |
| Theoryc | ⋯ | ⋯ | ⋯ | 2885.86 | ⋯ | ⋯ |
| Theoryd | ⋯ | ⋯ | ⋯ | 2872.98 | ⋯ | ⋯ |
| Theorye | 118.486 812(9) | 4161.1661(9) | ⋯ | ⋯ | ⋯ | ⋯ |
| $\mathrm{Exp}.$ | 118.486 84(10)f | 4161.1660(03)g, | ||||
| ⋯ | 4161.16632(18)h | ⋯ | 2885.98i |
aThis work for non-relativistic case. | |
bThis work for relativistic case. | |
cAnalytical calculation by extended Lennard-Jones potential, see [89]. | |
dCalculation by ab initio multi-reference configuration interaction calculation with the digit standing for diatomics (EHFACE2), see [90]. | |
eCalculation by nonadiabatic perturbation theory with considering relativistic quantum electrodynamic, see [91]; the experimental value: | |
fSee [92]. | |
gSee [93]. | |
hSee [94]. | |
iSee [90]. |