1. Introduction
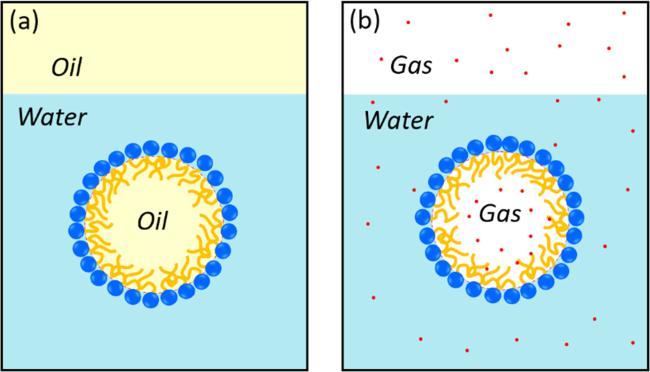
Figure 1. The similarities and differences between microemulsions and BNBs at small surfactant concentrations. (a) The oil-in-water microemulsion phase coexisting with an excess oil phase. (b) Nanobubble-containing aqueous solution that coexists with an excess gas phase. For the oil-in-water microemulsion, the oil droplet (colored in yellow) is encapsulated by a surfactant monolayer in a continuous phase of water, while for the BNB the gas bubble is encapsulated with a compressed amphiphile monolayer in the aqueous solution. In both cases, amphiphile molecules (or surfactants composed of a hydrophilic head in blue and a hydrophobic tail in orange) play an important role in their stability. |
2. Results and discussion
2.1. The similarity between BNBs and microemulsions
2.2. The compressed amphiphilic monolayer model and the stabilization mechanism of BNBs
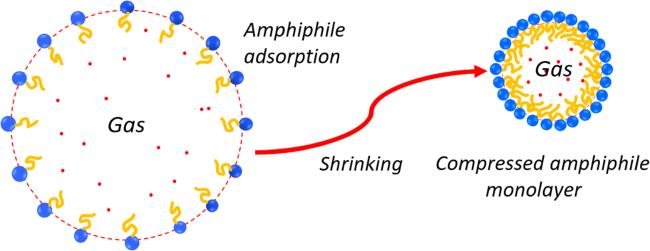
Figure 2. Schematic drawing of the formation of a compressed amphiphilic monolayer when a microbubble shrinks. The shrinking of a large bubble leads to a decrease in surface tension. Once a minimum in the average area occupied by each surfactant molecule is reached, a compressed amphiphile monolayer is formed. For the compressed amphiphile monolayer, the interfacial free energy for the nanobubble is dominated by the curvature free energy. |
2.3. The mechanical and chemical equilibriums of BNBs in three-phase coexistence
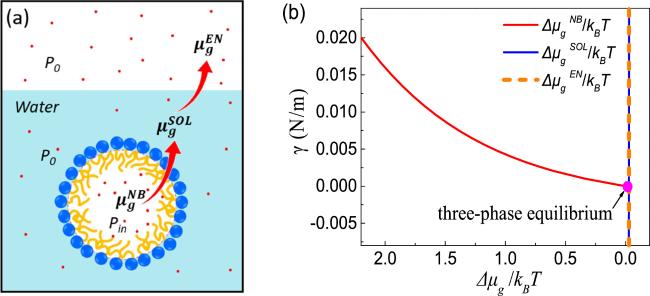
Figure 3. Schematic of three-phase equilibrium. (a) The chemical equilibrium of gas at different phases. ${\mu }_{g}^{{NB}},$ ${\mu }_{g}^{{SOL}}$ and ${\mu }_{g}^{{EN}}$ are the chemical potentials of the gas inside the bubble, in solution and in the environment, respectively. The chemical equilibrium for gas molecules requires ${\mu }_{g}^{{NB}}={\mu }_{g}^{{SOL}}={\mu }_{g}^{{EN}}.$ (b) The required gas-liquid surface tension for a BNB in mechanical equilibrium with its surrounding as a function of the chemical potential for gas inside the nanobubble, under the conditions of P0 ≈ 100 kPa and R ≈ 50 nm. For the purpose of simplification in the figure we assume that the gas in solution and the gas in the environment are in chemical equilibrium, namely, ${\mu }_{g}^{{SOL}}={\mu }_{g}^{{EN}}.$ The pink dot indicates the particular situation where the chemical potentials for the three phases are the same, i.e. three-phase coexistence. |
2.4. MD simulations validate that three-phase equilibrium can be reached
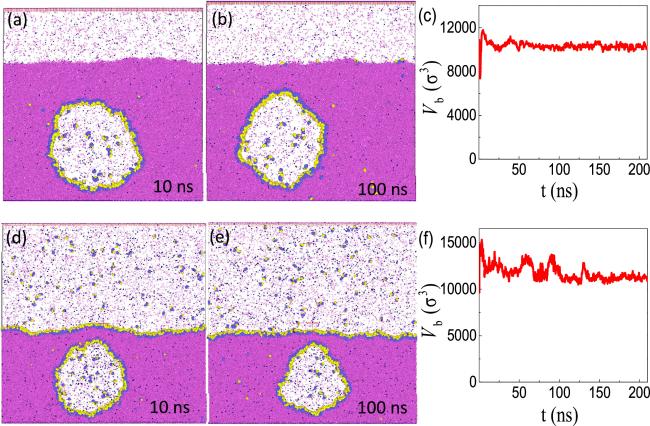
Figure 4. Verifying the three-phase equilibrium with MD simulation. (a), (b) Two typical snapshots for a BNB fully covered by H5T3 surfactant molecules. (c) The time evolution of the bubble size. In this case, the numbers of surfactant, liquid and gas molecules added are 492, 121 848 and 768, respectively. (d)-(f) A different situation in which both the bubble and the flat gas-liquid interface are covered by H5T3 surfactant molecules. In this case the numbers of surfactant, liquid and gas molecules added are 1092, 162 936, and 1952, respectively. |
2.5. Nanobubble size can be estimated from the rigidity of the compressed amphiphile monolayers
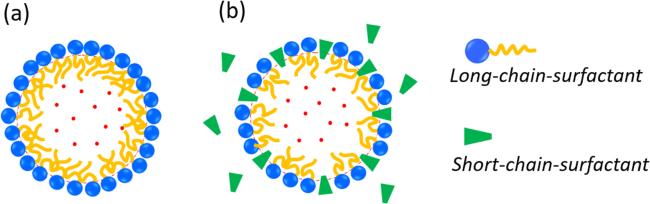
Figure 5. Schematic drawing of two different models for the compressed amphiphile monolayer: (a) the monolayer comprises only long-chain-surfactant molecules and (b) the monolayer contains both long-chain and short-chain surfactant molecules. |
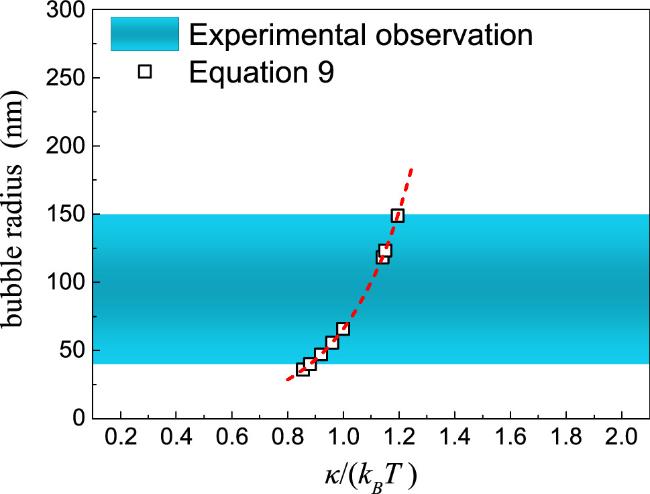
Figure 6. Comparison of predicted nanobubble radius (equation ( |