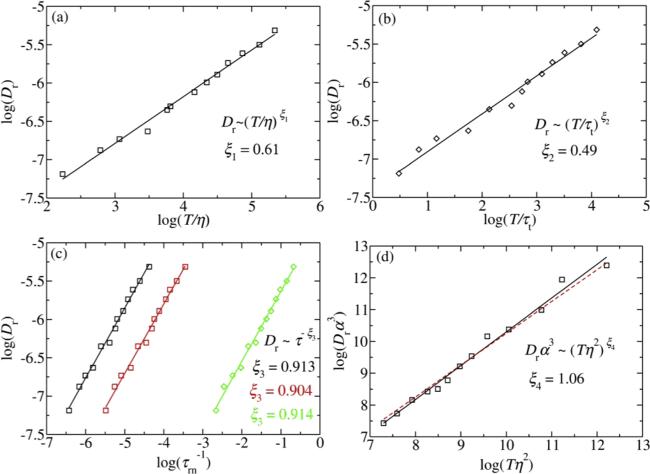
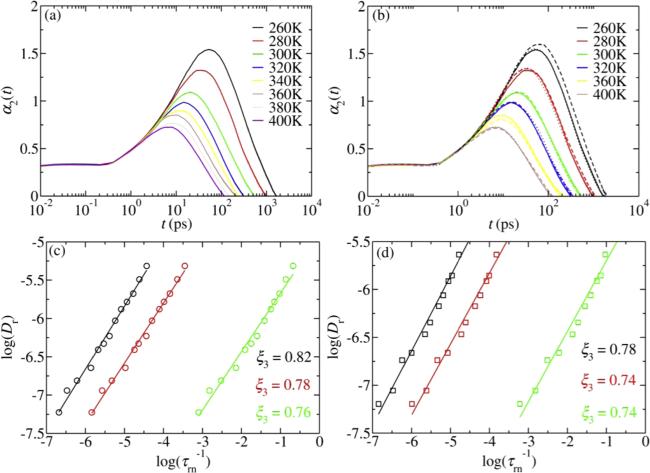
Many studies proposed the SED relation is invalid in supercooled liquids by testing with above variants. A deviation from ${\tau }_{r1}/{\tau }_{r2}=3$ is observed in both analytical models and experiments [
5–
7]. De Michele and Leporini [
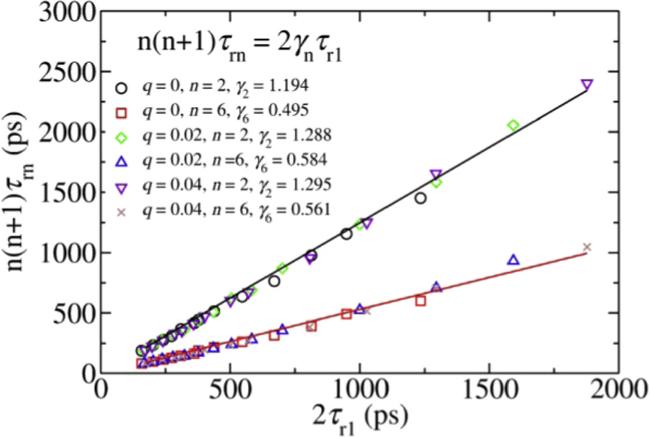
8] found the ratio $n\left(n+1\right){\tau }_{rn}/2{\tau }_{r1}\ne 1$ for $n=2,\,3,\,4$ in a supercooled liquid consisted of rigid dumbbells interacting via a Lennard–Jones potential. They found the $n\left(n+1\right){\tau }_{rn}/2{\tau }_{r1}$ and $n\left(n+1\right){D}_{r}{\tau }_{rn}$ firstly decrease to a minimum and then increase with deceasing temperature. The ${\tau }_{r1}{D}_{t}$ in a dumbbell model interacting via a repulsive ramp like potential [
9] is not a constant but temperature and density dependent. Similar phenomena are also observed in the supercooled SCP/E water [
10–
12]. The ratio ${D}_{r}{\tau }_{t}/T$ is almost a constant in supercooled SPC/E water [
4] within 280–350 K, but increases with cooling below 280 K. However, a fractional form ${D}_{r}\sim {\left(T/{\tau }_{t}\right)}^{\xi }$ with $\xi =0.75$ is observed for the whole temperature range. The ratio ${D}_{t}/{D}_{r}$ is found not to be a constant but is decreased with decreasing temperature. Kawasaki and Kim [
3] observed that ${D}_{t}/{D}_{r}$ is also decreased with cooling in TIP4P water; however, ${D}_{r}{\tau }_{rn}$ shows a reverse trend for $n=1,\,2,\,3,\,6.$ They found the ${\tau }_{r6}T/\eta $ is almost established but a fractional form ${\tau }_{r1}\sim {\left(\eta /T\right)}^{\xi }$ is observed with $\xi =0.8.$